GI bleed
Etiology
- The site of bleeding is only suggested by the patient’s presentation and physical examination.
- Stool can turn black (melena) with as little as 50-100 cc of upper GI bleeding
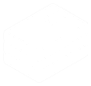
Pre-endoscopic clinical risk stratification
Clinical judgment always comes first.
- Assess the following features, based in part upon Rockall scale, BLEED criteria and Glasgow-Blatchford score.
*Major co-morbidity defined as CAD, CHF, renal failure, liver disease, sepsis, disseminated malignancy, altered
mental status, pneumonia, COPD, asthma.
- Use clinical risk to determine urgency of endoscopy and level of care required.
- Absolute indications for ICU admission:
- Shock
- Orthostasis
- Acute HCT decrease by >6%
- Transfusion requirements >2units pRBCs
- Active bleeding – ongoing hematemesis, bright red blood per NGT, hematochezia
- Most deaths are secondary to respiratory, cardiovascular, infectious, and renal complications associated with bleeding, not from exsanguination.
Evaluation
- Risk factors/Hx: NSAID use, alcohol use, liver disease/varices, severe retching, h/o GI bleed, past endoscopy, prior abdominal surgery, aortic aneurysms/grafts, trauma, coagulopathy, anticoagulation, malignancy
- Physical Exam:
- VS (with orthostatics)
- Rectal – note the color/consistency of stool (black/tarry = melena; bright red blood = hematochezia), palpable masses, external anal findings (e.g., hemorrhoids, fissures), smear stool on paper towel to see true color/content
- Guaiac testing is not useful to determine inpatient management
- Abdominal mass, peritoneal signs
- Stigmata of ESLD (asterixis, ascites, spiders, palmar erythema, caput, jaundice, testicular atrophy, gynecomastia)
- HEENT: epistaxis, telangiectasias (hereditary hemorrhagic telangiectasias, EtOH.)
- NG lavage is not required for upper GI bleeding patients. Endoscopist may use in certain patients to triage the timing of endoscopy but should not be placed routinely. It can be helpful in severe hematochezia patients to differentiate brisk upper GI bleed from lower GI bleeding.
- Coagulopathy and varices are not contraindications to NG tube placement.
- Note amount of aspirate obtained, the quality of the aspirate, and amount of saline lavage that is required to clear it.
- The lack of blood in the NG aspirate does not rule out an upper GI bleed as it may have only sampled gastric content and bleed may be duodenal
- Bile in the aspirate may confirm placement beyond the pylorus
- Low threshold to get KUB if peritoneal signs or abdominal distension. CT is more sensitive for free air, perforation, or ischemia.
- ECG for patients with a history of CAD or age > 45.
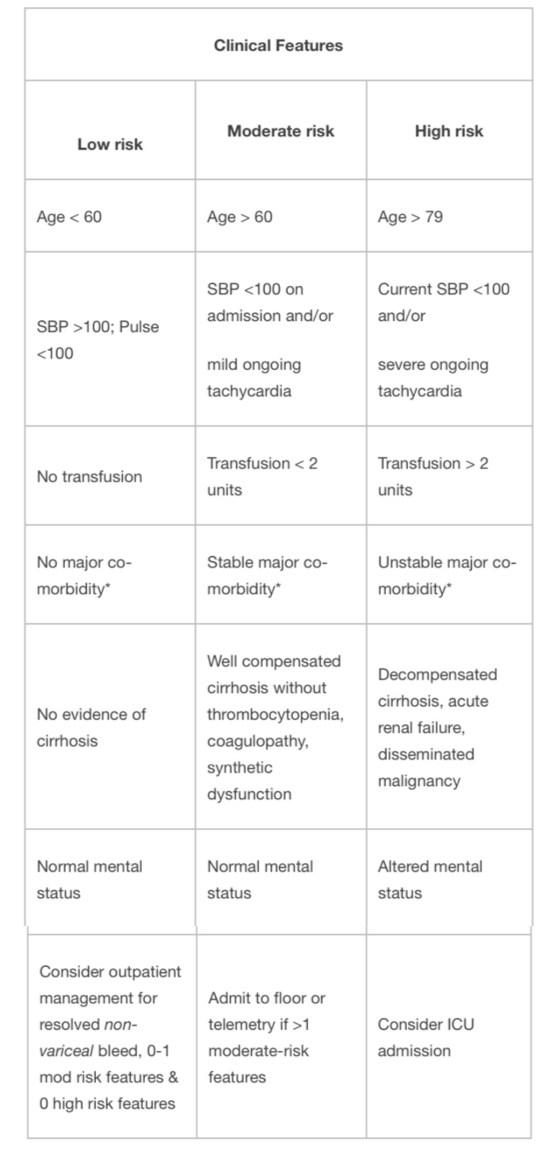
Management
Evaluation and Stabilization:
Common Management of Upper and Lower GI bleed
- NPO until cleared by GI
- Volume resuscitate with crystalloid.
- 2 large bore (16g or larger) PIVs.
- Consider cordis or rapid infusion catheter instead of triple lumen for central access.
- Most recent studies support restrictive transfusion strategies (goal Hg>7), though may consider goal of Hg>8 for patients with CAD.
- Protonix (bolus 80mg IV x 1 à 8mg/hr IV gtt)
- Reduces mortality in lesions subsequently seen to have high-risk stigmata for rebleed, reduces rebleeding after endoscopy, length of stay, and need for transfusion in patients with high-risk ulcer
- PPI before endoscopy: no difference in mortality, re-bleed rate, or need for surgery than if given after endoscopy, but decreases rate of high-risk stigmata of re-bleeding and need for subsequent endoscopic therapy
- Check H. pylori (serologies, endoscopic biopsy, stool antigen) and treat if present.
- H. Pylori diagnostic testing has higher false-negative rates in setting of bleeding (or PPI use.) Repeat if negative in setting of acute bleeding and clinical suspicion is high.
- Consider FFP, vitamin K if INR > 1.5, prothrombin complex concentrate (such as Kcentra), DDAVP (0.3 mcg/kg IV q12h x 2 doses) if uremic bleeding, and platelets if platelet count < 50. In ESLD patients, also order fibrinogen & replete with cryoprecipitate to >100.
- Consider massive transfusion protocol if hemodynamically unstable/requiring large amount of blood products.
- Complications of massive transfusion:
- metabolic alkalosis (2/2 citrate metabolism) in setting of renal impairment
- hypokalemia (precipitated by alkalosis)
- hypocalcemia (due to citrate binding)
- hypothermia
- hyperkalemia (particularly with older blood products)
- Avoid NSAIDs, ASA, anticoagulants
- Surgery consult if recent surgical procedure or rebleeding (>6u pRBC)
- Re-bleeding:
- Preferred treatment is repeat endoscopic therapy
- If patient has persistent bleeding after endoscopy, consider CT angiogram or IR embolization. For uncontrolled bleeding requiring >6u pRBC there is no significant difference (rates of re-bleeding, subsequent therapy, or mortality) for IR embolization vs. surgical intervention.
- Other diagnostic modalities (discuss with GI first)
- Tagged RBC scan (can detect bleed rate >0.1cc/min, localizes bleeding to area of abdomen but variable localization to portion of intestinal tract, can re-scan several times over 24-48h after tagged RBC administration for intermittent bleeding). If positive, consider IR embolization.
- CT (mesenteric) angiography (requires >1 cc/min.)
Specific management for suspected variceal bleed
- Goal hemoglobin > 7, plt >50, INR <1.5. More aggressive resuscitation may result in higher rates of re-bleed due to increase in portal pressures, particularly if Hb > 9.
- Octreotide 100 mcg bolus then 50 mcg/hr x 72 hours (constrict splanchnic circulation.)
- Discontinue PPI drip after endoscopy if there is no ulcer.
- Ceftriaxone (1gm q24 hrs x 5 days): reduces variceal re-bleeding, infection, and mortality in patients with cirrhosis +/- ascites.
- Endoscopy fails to control bleeding in ~10-20% patients.
- For persistent bleeding or re-bleed:
- Repeat endoscopic therapy
- Balloon tamponade (e.g. Sengstaken-Blakemore,tube): 50% rate of re-bleed upon balloon deflation, other complications including esophageal necrosis, rupture.
- TIPS
- Surgery
- Beta blockers: use in patients with proven variceal bleeds after the acute bleed has resolved and s/p octreotide x 72 hours. Nonspecific beta blockers (propranolol, nadolol) can be used as secondary prophylaxis against variceal re-bleeding .
- Titrate to a dose that lowers the baseline heart rate by 25% or to goal heart rate 55-60, as tolerated by the patient (15-30% of patients won’t tolerate due to low blood pressure.)
- Arrange for GI follow-up (endoscopy) for serial banding.
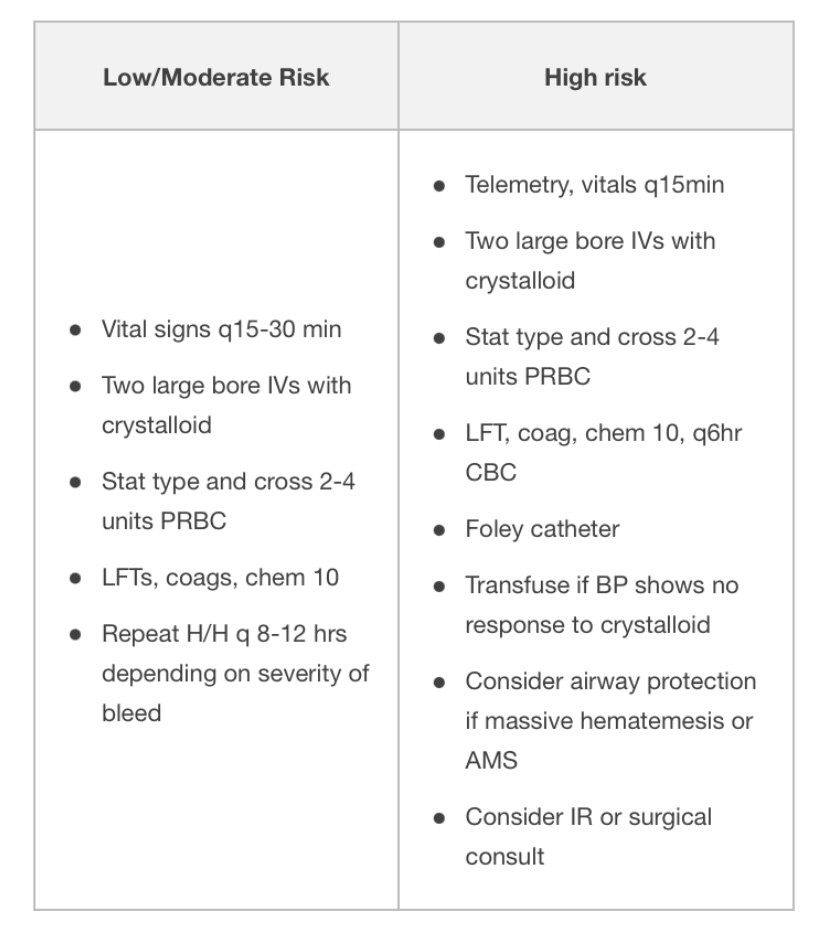
Endoscopy
- Diagnostic and therapeutic, as well as prognostic
- Correction of anticoagulation should not delay endoscopy
The following are endoscopic findings that a GI consultant uses to further risk stratify patients:
* Risk of (rebleeding, mortality rate)
- Length of observation for re-bleeding after endoscopy depends on endoscopic and clinical risk criteria. Rapid post-endoscopy discharge is reasonable for some patients with low-risk endoscopic findings, but discuss with GI first.
Key points
- Melena requires as little as 50-100 cc of blood and usually signals upper GI bleed, but can occur with light colonic bleeding with a slow transit time
- Most deaths occur from respiratory, cardiovascular, infectious, and renal complications associated with bleeding, and not exsanguination.
- NG tube lavage is not routinely required in all GI bleed patients
- The lack of blood in the NG aspirate does not rule out an upper GI bleed
Reference: Hospitalist Handook
No comments to display
No comments to display